The first and only FDA-approved topical gel for facial angiofibroma associated with tuberous sclerosis in adults and children 6 years and older

HYFTOR® (sirolimus topical gel) 0.2% is
an mTOR inhibitor in a clear gel.

Not actual patients. Individual results may vary.
The Only FDA-Approved Rx Option
HYFTOR® is indicated for the treatment of facial angiofibroma associated with tuberous sclerosis complex (TSC) in adults and pediatric patients 6 years of age and older. Facial angiofibroma is one of the most visible manifestations of TSC.1,2
Learn more about how FDA-approved HYFTOR® could help treat your patients’ Facial Angiofibroma.
The first and only FDA-approved topical gel for facial angiofibroma associated with tuberous sclerosis in adults and children 6 years and older

HYFTOR® (sirolimus topical gel) 0.2% is an mTOR inhibitor in a clear gel.
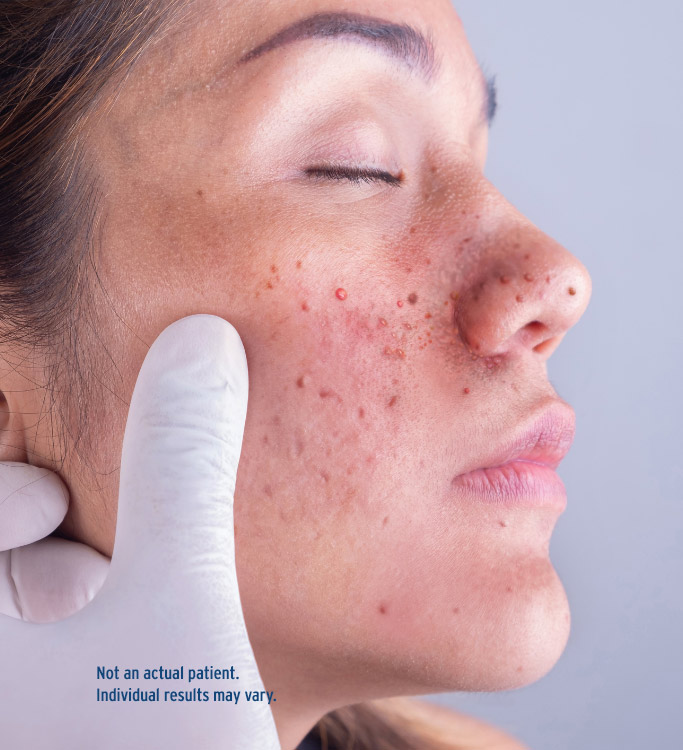
Patient Impact
Facial Angiofibroma: One of the most common and visible signs of TSC. About the impact TSC and facial angiofibroma can have on your patients.
HYFTOR® addresses an unmet need in the current treatment landscape.
HYFTOR® is the First “FDA-approved” Facial Angiofibroma Topical Treatment in the U.S.
HYFTOR® (sirolimus topical gel) 0.2% is commercially available in the U.S. by prescription as the first prescription topical treatment indicated for facial angiofibroma associated with tuberous sclerosis complex in adults and children 6 years of age or older.
FDA-approved, cGMP manufactured’ HYFTOR® is available through specialty pharmacies equipped to handle cold storage requirements and meet the unique needs of the small population of tuberous sclerosis complex (TSC) patients with facial angiofibroma and their providers.
Resource & Support
Resources are available to help answer common questions about HYFTOR®.
- Our expanded specialty U.S. nationwide pharmacy network list
- ICD-10 codes
- Dosage and Administration Guide
- Prescription-Pad-Fact-Sheet
- Prescribing Information
- Patient Brochure
Patient Assistance
“Nobelpharma Connect” strives to provide support and assistance to eligible patients in need. The Program is designed to help individuals with access and information throughout the treatment journey.
Contact Us
Whether you have HYFTOR®-related questions, need resources, or support starting a patient, our HYFTOR® Representatives are here to help you and your office staff.
You may also opt in to receive updates and the latest information regarding HYFTOR®.
- HYFTOR® Gel 0.2% Prescribing Information. Nobelpharma America, LLC; 2021.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154(7):781-788. doi:10.1001/jamadermatol.2018.1408.
Indication and Important Safety Information
Indication HYFTOR® is an mTOR inhibitor immunosuppressant indicated for the treatment of facial angiofibroma associated with tuberous sclerosis in adults and pediatric patients 6 years of age and older.
Important Safety Information
Contraindications HYFTOR® is contraindicated in patients with a history of hypersensitivity to sirolimus or any other component of HYFTOR®.
