 Facial Angiofibroma: One of the Most Common and Visible Signs of TSC
Facial Angiofibroma: One of the Most Common and Visible Signs of TSC
Tuberous Sclerosis Complex
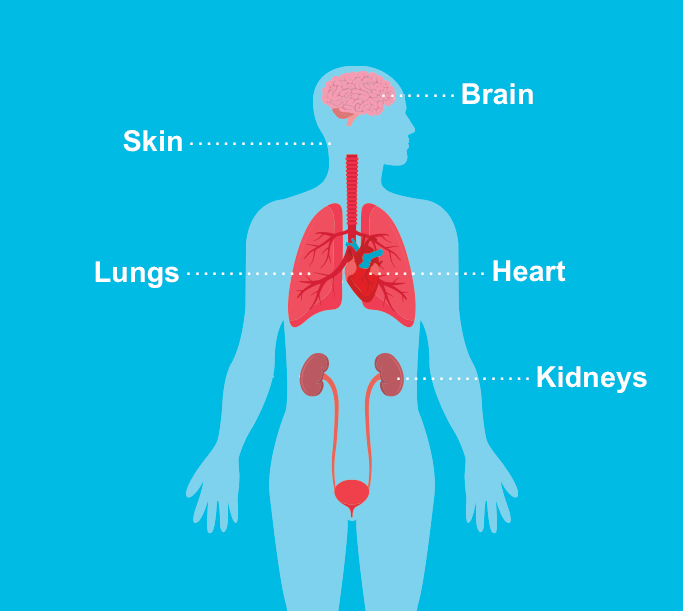
Tuberous sclerosis complex (TSC) is a genetic multisystemic disease that affects approximately 1 in 6,000 live births.1 It causes noncancerous tumors, or hamartomas, to form throughout the body. These benign tumors can develop in many organs, including the brain, eyes, kidneys, liver, lungs, heart, and skin.1
TSC is an autosomal dominant disorder. It is caused by abnormalities in the TSC1 and TSC2 genes. However, symptoms and severity may differ substantially—even among members of the same family. The distribution, number, size, and location of tumors can vary.1 TSC can be so mild that individuals go undiagnosed, or it can cause significant complications and may even be life-threatening.2
Symptoms and Complications1
- Approximately 50% of TSC patients had retinal hamartomas
- 70%-90% had renal abnormalities
Approximately 90% had symptoms of cerebral pathology - More than 90% had skin tumors
The skin is one of the most affected organs in people with TSC.3
Facial Angiofibroma
Facial angiofibromas are pink-to-red tumors typically located on the cheeks, nose, and chin, often appearing in a butterfly pattern over the malar eminences and nasolabial folds.1,4
Facial angiofibromas are made up of blood vessels and fibrous tissue and can coalesce to form patches. They can bleed, obstruct the nasal openings, and cause disfigurement.3,5
Facial angiofibroma occurs in approximately 75%-80% of TSC patients, making it the one of the most predominant skin manifestations of this disease.3-5
Impact of Facial Angiofibroma on Patients
Facial angiofibromas can appear in early childhood, becoming more numerous and larger as patients grow older.2 Facial angiofibromas initially cause a ruddy appearance on the cheeks. However, they eventually become rougher and cobblestoned.1
Left untreated, facial angiofibromas can cause disfigurement.5 These tumors may also impose a psychosocial burden on patients.3 Patients have reported that facial angiofibroma may have adverse effects on appearance and self-image, prompting some to avoid social situations.6
Unmet Need in the Treatment Landscape
These tumors are sometimes treated with invasive modalities. However, these procedures may require anesthesia. Invasive treatments can also be difficult to administer in patients with extensive angiofibroma or severe intellectual impairments.4
Interventional Treatments for Facial Angiofibroma Include:7
- Radiofrequency ablation
- Cryotherapy
- Electrocoagulation
- Dermabrasion
- Laser treatments
These treatments can be invasive and may require anesthesia.
- Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657-668. doi:10.1016/S0140-6736(08)61279-9
- National Organization for Rare Disorders (NORD). Tuberous sclerosis. Accessed August 25, 2020. http://rarediseases.org/rare-diseases/tuberous-sclerosis
- Cinar SL, Kartal D, Bayram AK, et al. Topical sirolimus for the treatment of angiofibromas in tuberous sclerosis. Indian J Dermatol Venereol Leprol. 2017;83(1):27-32. doi:10.4103/0378-6323.190844
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154(7):781-788. doi:10.1001/ jamadermatol.2018.1408
- Northrup H, Koenig MK, Pearson DA, Au KS. Tuberous sclerosis complex. July 13, 1999. Updated April 16, 2020. Accessed April 21, 2021. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington, Seattle; 1993-2021.
- Tuberous Sclerosis Alliance. Skin. Updated December 2013. Accessed June 8, 2020. https://www.tsalliance.org/about-tsc/signs-and-symptoms-of-tsc/skin
- Salido-Vallejo R, Garnacho-Saucedo G, Moreno-Giménez JC. Current options for the treatment of facial angiofibromas. Actas Dermosifiliogr. 2014;105(6):558-568. doi:10.1016/j.ad.2012.11.020
Indication and Important Safety Information
Indication HYFTOR® is an mTOR inhibitor immunosuppressant indicated for the treatment of facial angiofibroma associated with tuberous sclerosis in adults and pediatric patients 6 years of age and older.
Important Safety Information
Contraindications HYFTOR® is contraindicated in patients with a history of hypersensitivity to sirolimus or any other component of HYFTOR®.
