 Understanding TSC and Facial Angiofibroma
Understanding TSC and Facial Angiofibroma
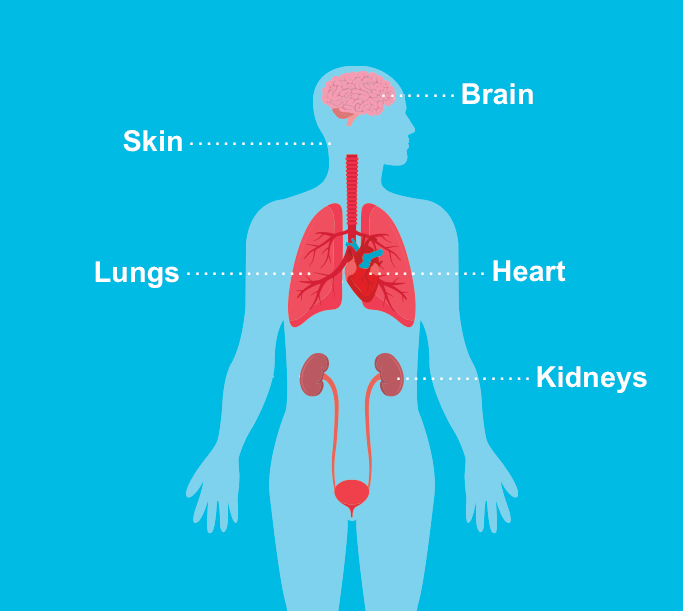
Tuberous Sclerosis Complex
Tuberous sclerosis complex (TSC) is a rare disease. Only 1 in 6,000 children born in the United States may have TSC. The disease causes benign (noncancerous) tumors to grow throughout the body. These tumors can develop in many organs, including the brain, eyes, kidneys, liver, lungs, heart, and skin.
Genetic and Variable
TSC is a genetic (inherited) disorder. It is caused by defects in 2 genes called TSC1 and TSC2. The symptoms and severity of TSC may vary widely—even among members of the same family. The tumors can grow in different places, in different numbers, and in different sizes. TSC can be so mild that some people are never diagnosed with it, or it can cause serious complications and even be life-threatening.
Symptoms and Complications
- Approximately 50% of TSC patients had retinal (eye) tumors
- 70%-90% had renal (kidney) abnormalities
- Approximately 90% had symptoms of cerebral pathology (brain conditions)
- More than 90% had skin conditions
The skin is one of the most affected areas in people with TSC.
Facial Angiofibroma
Facial Angiofibromas are pinkish or reddish bumps that are usually located on the cheeks, nose, and chin. Although they can appear in other areas of the skin, facial angiofibromas often appear in a butterfly pattern across the nose and cheeks.
Facial Angiofibromas are made up of blood vessels and tough tissue, and they can build up into larger patches. Although facial angiofibromas are benign, they can bleed, block the nasal openings, and cause disfigurement.
Impact of Facial Angiofibroma
Growth Over Time
Facial Angiofibromas can appear in early childhood. Then they can increase and grow larger as people age. At first, facial angiofibromas can cause a reddish or rosy appearance on the cheeks. However, they eventually become rougher and thicker.
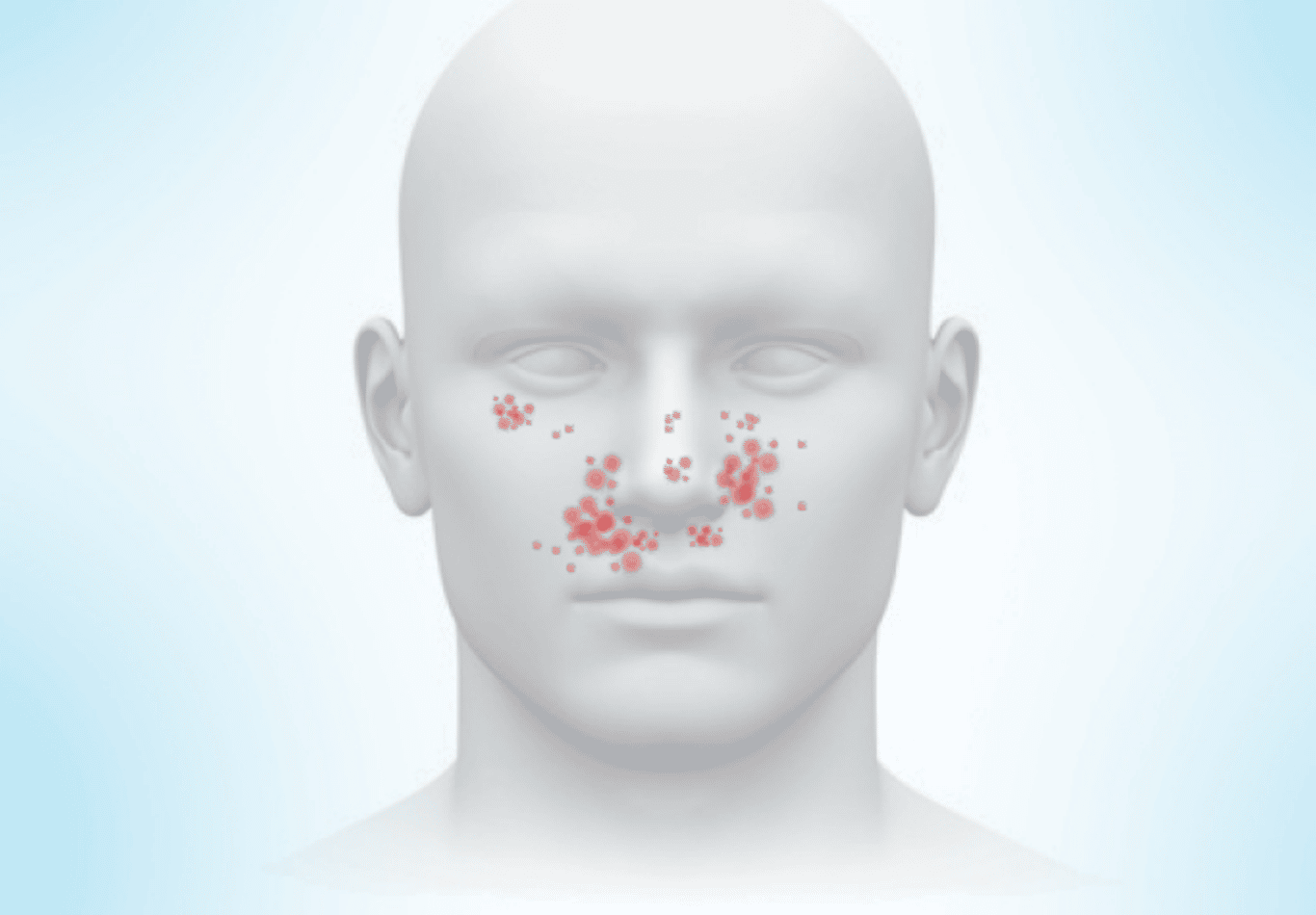
Facial angiofibroma occurs in approximately 75%-80% of TSC patients—making facial angiofibroma one of the most common skin conditions seen in TSC.
Psychological and Social Impact
Facial Angiofibromas can cause disfigurement if left untreated. These tumors may also be a psychological and social burden for people living with this condition. Patients have reported that facial angiofibroma can have negative effects on appearance and self-image, causing some people to avoid social situations.
When people look at a face, they typically focus on features, such as the eyes, nose, and mouth. But when a facial deformity is present, people may focus on the blemish. Facial Angiofibromas can lead to appearance concerns, and may have psychosocial impacts.
Invasive Treatment Options
Although some patients are successfully treated with these procedures, they may require anesthesia. Invasive treatment may include:
- Surgical removal
- Laser therapy
- Dermabrasion
Patients with Facial Angiofibroma May Experience
Questions, Concerns, and Advice from Others
Fear of Going to School / Work
Developmental Delays
Bullying
Behavioral Issues / Adjustment Disorders
Lack of Confidence
Appearance / Self-image Issues
Important Safety Information
What is HYFTOR®? HYFTOR® is a prescription medicine that is used on the skin (topical) to treat adults and children 6 years of age and older with a type of noncancerous tumor called angiofibroma on your face caused by the genetic condition tuberous sclerosis. It is not known if HYFTOR® is safe and effective in children under 6 years of age.
Important: HYFTOR® is for use on the skin only (topical use). Do not use HYFTOR® in your mouth, eyes, or vagina.
